|
Types of steel
Steel is basically an alloy of the elements iron (Fe) and carbon (C), where the latter represents up to 2% of the total mass. Even a very small amount of carbon considerably changes the mechanical properties of steel, particularly improving its strength, which is crucial for any practical application. An alloy with more than 2% of carbon is called cast iron, quite a brittle material and hence unsuitable for kitchen knives or similar tools. However, a high carbon content results in a lower melting point, which means that cast iron is easier to pour into molds and thus, for example, suitable for making cast iron pots.
Steel can also be mixed with other elements, often chromium (Cr), vanadium (V) and molybdenum (Mo), which further improve its mechanical properties and, in some cases, also corrosion resistance. This group is called alloy steel. A special subgroup of alloy steels are tool steels, the common feature of which is that they are used for tools (knives, saws, axes, drills etc.). They are suitable for use whenever high toughness, strength and abrasion resistance are required.
The exceptional mechanical properties of tool steels consequently mean that they are demanding to produce and process, which in turn makes them more expensive compared to steels with fewer alloying elements. In terms of material, most quality kitchen knives belong to the family of tool steels. The figure below shows a schematic distribution of iron alloys and the families of steels used to make kitchen knives are highlighted. 👇
 |
Mechanical properties of materials
In order to describe the properties of kitchen knives and their differentiation in terms of quality, it is useful to first define some basic concepts regarding the mechanical properties of the material and how we measure them.
Mechanical properties: STRENGTH, HARDNESS, DUCTILITY, TOUGHNESS
➨ Strength
One of the most basic properties of metallic materials is their strength, defined as resistance to changes in shape under the influence of external forces. It is measured experimentally by tensile tests, where a sample of an elongated material is clamped in the jaws, which are then slowly pulled apart until the sample ruptures. In doing so, the curve of force as a function of jaws displacement is recorded. To make it easier to compare samples of different sizes, the values are usually converted into a stress versus strain curve. An example of this is shown in the figure below, which also schematically presents the typical external appearance of a sample. 👇

|
The initial, very steep part of the curve represents an elastic deformation when the material returns to its original shape as the load is removed. With a further increase in load, an irreversible change in shape occurs, i.e., the material is plastically deformed. We definitely want to avoid this situation with kitchen knives, because in practice it means that the cutting edge or the entire blade bends. The sample in the tensile test can be further stretched to a certain point where the maximum force is recorded, called “tensile strength”. After this point, the force even slightly decreases due to the transverse deformation of the sample, until the moment when the sample breaks.
➨ Hardness
The hardness of a material is, by definition, its resistance to embossing or localized plastic (permanent) deformation. Consequently, this also means resistance to wear. Hardness is a different quantity from strength, although they are directly related. Strength is physically more precisely defined, but hardness is usually easier to measure in practice and is also more relevant in the case of kitchen knives. There are several different methods of measuring hardness and they are based on pressing a standard-shaped probe into the surface of the material and measuring the depth of the impression. For tool steels, the Rockwell method (HRC) of hardness measurement is often used, where the probe is a diamond cone. However, there are other methods that are more suitable for softer materials, for example measuring hardness with a ball-shaped probe.
➨ Ductility
A relevant mechanical property is also ductility or plasticity, i.e., a measure of plastic deformation before fracture. In the above figure of the tensile test curve, this means the amount of deformation at point F, whilst the stress at which the rupture occurred is irrelevant.
➨ Toughness
Toughness, however, is the property of a material to absorb a lot of energy before it breaks. This means that it must withstand as much elongation as possible at maximum force. Some materials break at high force but at low elongation. We say they are brittle. The area under the tensile test curve represents toughness and is also shown in the figure below. 👇
 |
The mechanical properties of a metallic material depend on its chemical composition and thermo-mechanical treatment. The chemical element that has the greatest effect on the hardness of steel is carbon, while chromium, manganese, vanadium and molybdenum also positively affect hardness. Together with carbon, the latter elements form new, extremely hard compounds called carbides. |
Atomic structure of metals
 |
Metals are crystalline materials, which means that their atomic structure is arranged in unit cells. They are also said to exhibit a long-range order – they have a periodically repetitive structure over many interatomic distances. Different metals (metallic elements) have different types of unit cells that can even change with temperature. In the case of iron alloys, the two most important types of unit cell – face-centered cubic cell (left) and body-centered cubic cell (right) – are shown in the figure above. 👆
In practice, ideal crystals, where the same atomic structure would be periodically repeated without error over a long distance (e.g., the whole product), do not exist. In an ideal crystal structure, all crystals or metals contain defects of various types: point, line, planar, or volume defects.
Even in a chemically pure metal (metallic element), the crystal lattice contains point defects, which means that a particular atom is missing at its theoretical location or is inserted into the wrong location. The number of such errors increases exponentially with temperature. At a sufficiently high temperature, the atoms rapidly change places, move along the crystal lattice, and the number of defects increases until the ordered structure disintegrates. At that moment, the metal liquefies.
The two images below schematically represent examples of point errors: an atom of the same type disappears from a place in the crystal lattice where it should theoretically be located (figure left); or the atom of another element is inserted into the crystal lattice in an unexpected place or it replaces the atom of the majority element (figure right). 👇
 |
Line defects called dislocations are also always present in metals and they occur when one layer of atoms is inserted between other layers. Under the influence of external stresses, atoms belonging to the interlaced layer can switch their neighbors and establish a bond with other layers of atoms. In this way, the dislocations move along a metal lattice, thus allowing many atoms to permanently change their place. The movement and formation of new dislocations is a very important concept in metallurgy, as at the microscopic level it represents an explanation for the plastic deformation observed at the macroscopic level. This also leads us to the conclusion that, if we want to reduce the plastic deformation of our product or increase its strength, we must in some way inhibit the movement of dislocations. 👇
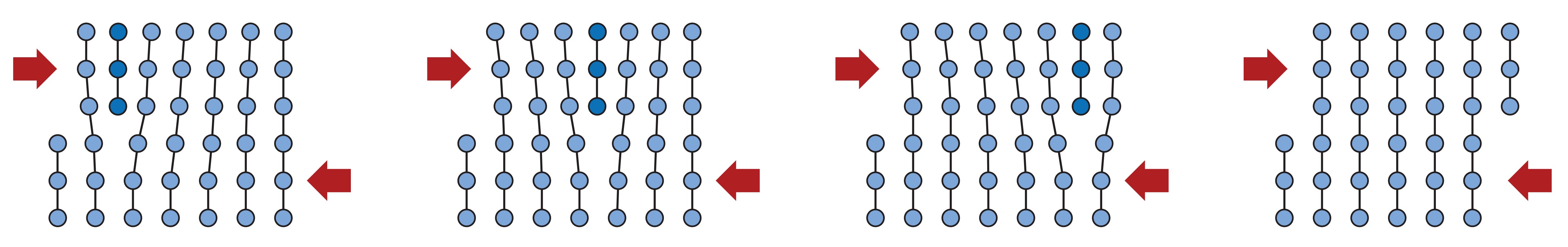 |
The theory of dislocations was established as early as the beginning of the 20th century and was experimentally unequivocally confirmed only some 50 years later with the invention of the electron microscope. The image below is an electron microscope image of dislocations on the crystal surface. |
Metal products do not have a crystal lattice that is uniformly oriented throughout their volume, but are rather composed of a large number of crystal grains with different orientations. This is due to the process of solidification of metal from liquid to solid state, which begins in many places at the same time. Differently oriented crystal grains in liquid metal grow outward until they collide with their neighbors and all the metal turns into a solid state. The crystal grains are not visible to the naked eye, they are typically no larger than a tenth of a millimeter, but they can be observed with an optical microscope. The two images below show a metallic microstructure with many crystal grains in the undeformed state (figure left) and after significant plastic deformation (figure right), when the crystal grains have changed their shape, i.e., they flattened. 👇
 |
Metal strengthening mechanisms
In previous chapters, we learned more about the basics of the atomic structure of metallic materials, definitions of important mechanical properties, and methods of measuring them. Now we can put this information together and determine which metallurgical processes can improve the strength and hardness of quality kitchen knives. |
A common feature of all strengthening mechanisms is that they hinder the movement of dislocations along the crystal lattices of metallic materials. On the microscopic scale, the movement of dislocations represents the mechanism of plastic deformation that is detected with the naked eye on the macroscopic scale.
➨ Strain hardening
Strain hardening is a phenomenon where the plastic deformation limit of a material increases with increasing plastic deformation. This is due to the formation of a vast number of new dislocations that travel in different directions along the crystal lattice and hinder each other’s movement. In the manufacturing of kitchen knives, this phenomenon occurs in the forging process, when the blades are plastically deformed or changed in shape by hammer blows. In practice, the process cannot be continued indefinitely, because, in addition to strength, brittleness also increases and the product can crack if there is too much plastic deformation.
The figure below shows the strain hardening curves as a function of the degree of plastic deformation for some typical materials. We see that the change in the limit of plasticity can be quite pronounced. 👇
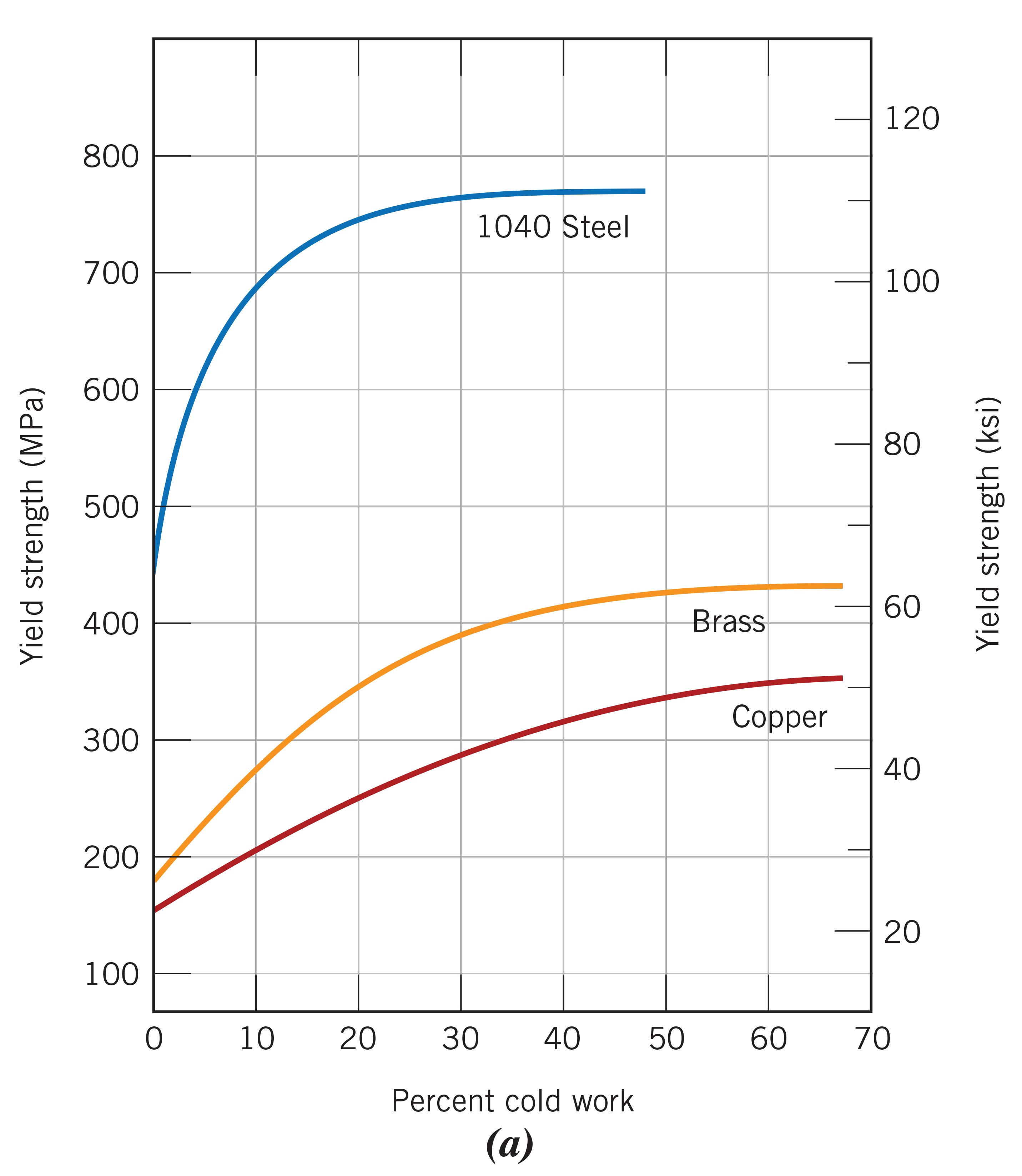 |
➨ Solid solution strengthening
Solid state hardening is a mechanism that explains why alloys of different elements are stronger than pure metals. The atoms of the elements added to the majority element are inserted into its crystal lattice, introducing irregularities because of variously sized atoms. Irregularities in the crystal lattice cause internal stresses, which in turn obstruct the movement of dislocations. This is also shown schematically in the figure below. This mechanism explains why steel, which is an alloy of carbon and iron, is stronger than pure iron and why alloying with additional elements (Cr, Mo, V) further improves its mechanical properties. 👇
 |
➨ Strengthening with grain size control
At the microscopic level, metal products consist of a large number of crystal grains that are randomly oriented. The ordered sequence of atoms in one crystal grain does not continue across the boundary in the other crystal grain. Therefore, the boundaries of the crystal grains represent obstacles to the movement of dislocations and consequently also prevent plastic deformation (picture below). Crystal grains typically occur in the size range of 0.001-0.1 millimeters. The smaller the grains, the more boundaries between them per unit of volume and the more they hinder the movement of dislocations. This hardening mechanism explains why kitchen knives with a fine-grained structure, such as Japanese knives, are stronger and of better quality. The grain size in the product is dependent on a complex combination of the effects of chemical composition and thermo-mechanical treatment (e.g., hot forging). 👇
 |
➨ Quenching
Quenching is the process of improving mechanical properties by rapidly cooling a hot product. The first condition for the ability to harden is the existence of pure metal in two types of crystal lattices at different temperatures. At room temperature, iron exists in a body-centered cubic crystal lattice that at about 730 degrees Celsius turns into a face-centered cubic lattice. The same transition occurs in the opposite direction when the temperature decreases. The second condition is the presence of an alloying element, the atoms of which are evenly distributed in their characteristic places in the crystal lattice. In the case of steel kitchen knives, the base metal is iron and the alloying element is carbon. When the product cools down quickly enough from a high temperature (above 730 degrees Celsius), the iron atoms bond into another type of crystal lattice, while the carbon atoms do not have enough time to move to other places. They remain “frozen” in their previous places and introduce internal stresses into the crystal lattice, which in turn hinders the movement of dislocations.
The internal stresses caused by quenching can be so great that the product significantly changes shape, bends, or even cracks. This mostly depends on the chemical composition (percentage of carbon and other elements) and the cooling rate, which is controlled by the choice of a cooling medium (water, oil or air).
Conclusions
In the first chapter, we looked at how steels are classified according to general chemical composition and intended use, and which of them are used for quality kitchen knives. In the second chapter, we introduced the definitions of relevant mechanical properties of metallic materials and the principles of their measurement. This was followed by a quick overview of the atomic structure of metals and the connection between plastic deformation at the microscopic and macroscopic levels. When using kitchen knives, we want to prevent plastic deformation, because in practice it translates into damage to the cutting edge and reduced sharpness. In the last chapter, we combined all previous knowledge and presented metallurgical mechanisms that improve the strength of metallic materials. What they all have in common is that, at the microscopic level, they prevent the movement of dislocations in the crystal lattice in various ways. It is also important to note that all the described hardening mechanisms eventually fail at elevated temperatures because all atoms and consequently dislocations move faster. This also explains why quality kitchen knives should not be exposed to high temperatures for longer periods of time (for example above 150 degrees Celsius).
⬌
Glossary of terms:
➨ Alloy steels: steels which, in addition to carbon (C), also contain other elements, often chromium (Cr), vanadium (V) and molybdenum (Mo). These further improve its mechanical properties and, in some cases, also corrosion resistance.
➨ Strength: resistance to changes in shape under the influence of external forces.
➨ Tensile strength: the maximum stress that a material can withstand while being stretched or pulled before breaking.
➨ Hardness: resistance of material to embossing or localized plastic (permanent) deformation. Consequently, it also means resistance to wear.
➨ Rockwell method (HRC): a hardness scale used in metallurgy for measuring the hardness of hard substances. The result is a dimensionless number. There are two versions (and units) of this method: HRb and HRc.
➨ Ductility: the degree to which a material can sustain plastic deformation under tensile stress before failure. The more deformation a material can withstand without breaking due to brittleness, the more ductile it is.
➨ Toughness: the property of a material to absorb a lot of energy before it breaks.
➨ Carbides: binary compounds composed of carbon and a metal or, in some cases, a semi-metal. They have high strength and are brittle.
➨ Crystalline materials: solid materials whose constituents, such as atoms, are arranged in highly ordered microscopic structures or basic cells. These cells are periodically repeated in a three-dimensional crystal lattice and have symmetrical properties.
➨ Dislocations: a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. They are most relevant as regards metallic materials because they allow plastic deformation at a relatively small stress.
➨ Strain hardening: strengthening of a metal by plastic deformation. This strengthening occurs because of dislocation movements and dislocation generation within the crystal structure of the material.
➨ Forging: the shaping of metal where plastic deformation is caused by delivering consecutive blows with a hammer or by slowly applying a continuous pressure in a press.
➨ Solid state hardening: a mechanism that explains why alloys of different elements are stronger than pure metals.
➨ Quenching: a type of heat treating where steel is first heated to a quenching temperature (the temperature of austenite, a solid solution of iron, with an alloying element) and then cooled rapidly, thus obtaining martensite, a very hard form of steel crystalline structure.
⬌
Author of the article: Matevž Pintar, MSc in mechanical engineering
Images: Callister, William D. in Jr., Rethwisch, David G. 2014. Materials Science and Engineering: An Introduction. Hoboken: John Wiley & Sons, Inc.
